
STRUCTURE OF GRAPHENE
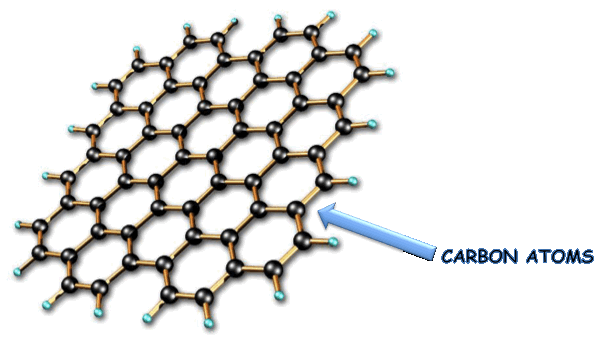 The
carbon
atoms,
in the structure of GRAPHENE, are arranged in a hexagonal
lattice,
as can be seen in the figure.
In
the structure of graphene the carbon
atoms are
sp2
hybridized
and
in
this type of hybridization, the carbon atoms can bond in order to
form a two-dimensional
honeycomb crystal;
the
length of the “C-C” bond is equal to 0,142 nm.
The
carbon
atoms,
in the structure of GRAPHENE, are arranged in a hexagonal
lattice,
as can be seen in the figure.
In
the structure of graphene the carbon
atoms are
sp2
hybridized
and
in
this type of hybridization, the carbon atoms can bond in order to
form a two-dimensional
honeycomb crystal;
the
length of the “C-C” bond is equal to 0,142 nm.
HYBRIDIZATION OF CARBON ORBITALS
The hybridization can be defined as a process in which a certain number of atomic orbitals (s, p, d) of one atom are combined each other. The atomic orbitals, involved in the hybridization process, have an energy content slightly different (valence orbitals). The combination of different atomic orbitals allows obtaining new hybrid, equivalent (iso-energetic) atomic orbitals. The hybrid atomic orbitals have lobes that are oriented along the directions of the possible bonds that one atom can form with other atoms. To explain the phenomenon of hybridization it is necessary to make reference to the electronic configuration that in this case involves the carbon atom.
A carbon atom has six protons and six electrons, two electrons occupy the “1s” atomic orbital and they are strongly bound; instead, two of the other four electrons (the valence electrons, which are located in the second energy level) are paired and occupy the “2s” atomic orbital; the other two electrons are unpaired and occupy different “2p” atomic orbitals; then the electron configuration of the carbon atom can be rewritten in this form: 1s22s22px2py.
The “2s” and “2p” atomic orbitals of the carbon atom have very similar energies, therefore the electrons belonging to them can move very easily, generating, in this way, the hybridization phenomenon.
The figure shows the schematic representations of the valence electrons of the carbon atom in the three kinds of hybridization; each representation corresponds to a different combination of the atomic orbitals of the second energy level. The exponent of the “p” letter indicates the number of the “p-type” atomic orbitals involved in the formation of the hybrid:
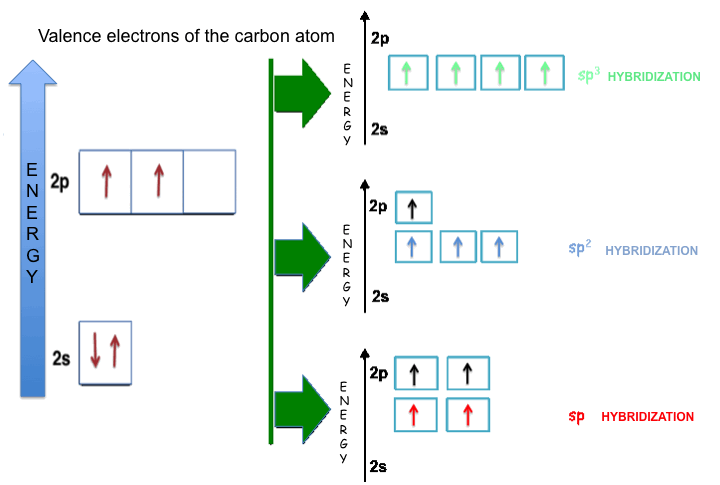
SP2 HYBRIDIZATION
In the particular case of sp2 hybridization, the 2s atomic orbital and the two atomic orbitals 2p (2px and 2py) are "mixed"; the result is the formation of three hybrid atomic orbitals called sp2 atomic orbitals. These atomic orbitals are iso-energetic, perfectly equivalent, oriented symmetrically in a plane; they form angles of 120° and give rise to the so-called planar triangular structure; the fourth atomic orbital “pz”, that is not involved in the hybridization, has the lobes perpendicular to the plane on which the three sp2 hybrid atomic orbitals are located:
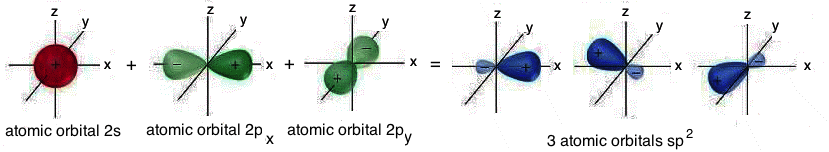 Graphic
representation of the sp2
hybrid
atomic orbitals.
Graphic
representation of the sp2
hybrid
atomic orbitals.
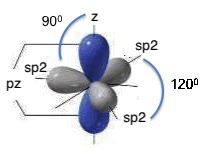 sp2
hybridization.
sp2
hybridization.
When two sp2 hybridized carbon atoms come close, they form a strong σ bond by overlapping of the sp2 – sp2 hybrid atomic orbitals; in this case also the p atomic orbitals, not hybridized but still present in each carbon atom, come closer so that they overlap and form a π bond, as can be seen in the figure:
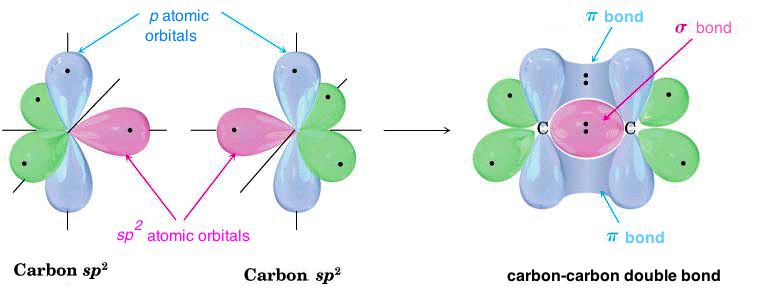
In sp2 hybridization each atom has three σ bonds available on the x-y plane, so that it forms a honeycomb lattice; in this lattice the pz atomic orbitals come together in a π bond, located above and below the x-y plane. This is the case of graphite, where many two-dimensional honeycomb lattices are held together, one on the other, by weak interaction forces, called “Van der Waals” forces.
The bond formed by sp2 atomic orbitals originates the nano-structures: fullerenes, nanotubes and graphene.
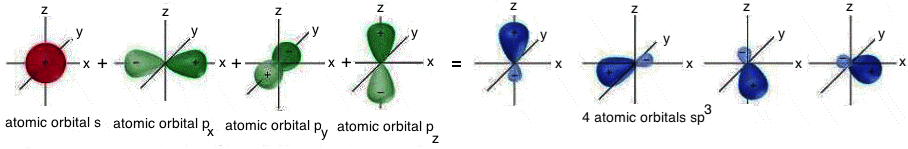
SP3 HIBRIDIZATION
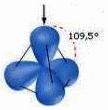
In the case of the diamond, every atom is bonded to other four atoms, placed on the vertices of a tetrahedron, this is called “sp3 hybridization”:
 Graphic
representation of the sp3
hybrid atomic orbitals.
Graphic
representation of the sp3
hybrid atomic orbitals.
 sp3
hybridization.
sp3
hybridization.
In sp3 hybridization there is the "mixing" between the 2s and the three 2p atomic orbitals. The result is the formation of four hybrid iso-energetic atomic orbitals, perfectly equivalent and symmetrically oriented in space; they form angles of 109,5° that correspond to the tetrahedral structure.
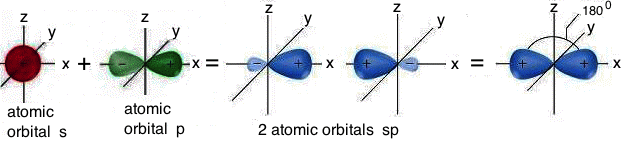
SP HIBRIDIZATION
The combination of a s with a p atomic orbital produces two hybrid atomic orbitals of sp type. In sp hybridization, the two hybrid atomic orbitals are iso-energetic, perfectly equivalent and they are symmetrically oriented on a line, so that they form an angle of 180° (linear structure). The two p type atomic orbitals, not involved in the hybridization, are arranged on planes perpendicular to each other and perpendicular to the line on which the two hybrid atomic orbitals of sp type are located:
 Graphic
representation of the sp hybrid
atomic orbitals.
Graphic
representation of the sp hybrid
atomic orbitals.
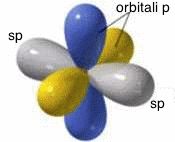 sp
hybridization.
sp
hybridization.
In all types of hybridization, the biggest lobe of the hybrid atomic orbital is involved in bonds with other atoms; for this reason, for simplicity, in the figures that represent the different types of hybridization, the smaller lobe has been omitted.