
DISCOVERING GRAPHENE
British people, in the sixteenth century, were the first to introduce the use of graphite. They believed that graphite was a lead-based material and initially they used it to mark the sheep. In the eighteenth century, graphite was identified appropriately and it was named by the current name.
The name “graphite” comes from the greek language "graphos", which means "to write”.
Graphite is a “lamellar mineral”; it is composed of many layers of carbon atoms, arranged in a two-dimensional hexagonal structure; each layer has a thickness of a single atom. Weak forces, said "Van der Waals" forces, hold together the layers of carbon atoms. Graphite can be imagined as a book composed of many sheets, each sheet corresponds to one carbon atoms layer.
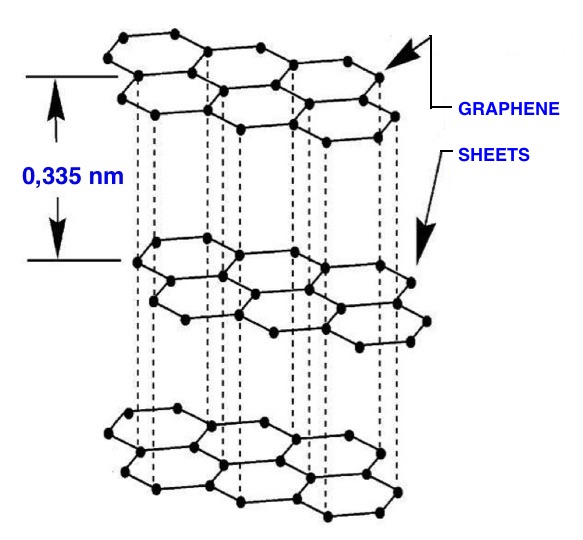 Graphite
structure
Graphite
structure
The single layer of graphite is known as “graphene”. The interest in graphene was born in the 1940s, when theoretical physicists began to study its properties; but they were not able to observe it. Over the years, the production of graphene, in the laboratory, did not get good results; therefore the existence of this material was questioned.
GRAPHENE was discovered in 2004 by the Russian physicists Andrej Konstantinovič Gejm and Konstantin Sergeevič Novosëlov, from the University of Manchester in England.
The two scientists discovered this material by accident, using the carbon graphite flakes, precisely those present in a common pencil, to investigate electrical properties of matter. In order to get thinner and thinner flakes of graphite, the two physicists used an extremely simple but very effective method, that it is known as "scotch-tape method” and based on the mechanical exfoliation of the graphite. In practice, a simple scotch tape was used, to rip thin layers from a piece of graphite. The technique of the mechanical exfoliation, repeated continuously for about twenty times, on the same fragments of graphite, allowed them to obtain layers of infinitesimal thickness; the two scientists put them on a substrate of SiO2 that was analyzed. The analysis of these thin layers showed that some of them had the thickness of a single atom: practically the "graphene" had been isolated.
 Graphite
flakes, deposited on scotch-tape
Graphite
flakes, deposited on scotch-tape
Gejm explained that one monoatomic layer of any material, could not exist theoretically, because: "we live in a 3D world, so that the 2D structures, such as graphene, could not be formed, they tend to distort and create 3D structures, such as graphite, fullerenes and nanotubes. But graphene was created in a different way, it was obtained by graphite".
In other words, it is impossible to create the graphene by the aggregation of single carbon atoms, but it is possible to obtain a layer of graphene already formed, starting from a block of graphite.
It took approximately one year to convince the international scientific community about the existence of graphene. Andrej Gejm and Konstantin Novosëlov, just for having highlighted the interesting properties of this material, obtained in 2010 the Nobel Prize in Physics.
 Andrej
Konstantinovič Gejm
Andrej
Konstantinovič Gejm
 Konstantin
Sergeevič Novosëlov
Konstantin
Sergeevič Novosëlov
Nowadays, the exfoliation of graphite can be made by elaborate mechanical methods, but also by using chemical methods; this has allowed the realization of graphene inks. These graphene inks could be used in the printing of opto-electronic circuits on easily rollable plastic substrates. The graphene inks are revolutionizing the Electronics world, currently oriented towards the “Flexible Electronics”. The electrical circuits could be obtained using two-dimensional materials - 2D, such as graphene and they could be used in advanced and innovative technologies. For example:
smartwatches, devices that could be adapted to our wrist and have functions extended to the biomedical field;
flexible displays that could be rolled up;
electronic circuits, inserted in the clothing, that could detect the state of our health.
|
Look at these videos and find out more: |
|
Read more: Andre Geim Graphene Revolution |