
CARBON
![]() Carbon
is
one of the most important among the chemical elements; the organic
compounds,
formed
by carbon, outnumber all the other chemical compounds.
Carbon
is
one of the most important among the chemical elements; the organic
compounds,
formed
by carbon, outnumber all the other chemical compounds.
The atomic number of the carbon is Z = 6, it is the number of protons in an atomic nucleus and it is equal to the number of electrons in a neutral atom; the electron configuration of the carbon is:
1s22s22p2
The graphic representation of the carbon electron configuration, namely the distribution of the electrons within the different energy levels of the atomic orbitals, is the following:
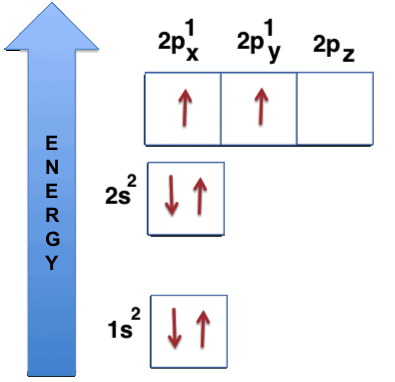 In
this figure the
atomic orbitals
are represented with squares, while the electrons are represented
with the arrows inside the squares; the direction of the arrows shows
the spin status of each electron.
In
this figure the
atomic orbitals
are represented with squares, while the electrons are represented
with the arrows inside the squares; the direction of the arrows shows
the spin status of each electron.
The electron configuration of the atoms, in particular the electron configuration of the carbon atom, can be explained in accordance to:
Table 1, which provides the values of the quantum numbers of the atomic electrons and the number of electrons that each energy level and sub-level contains:
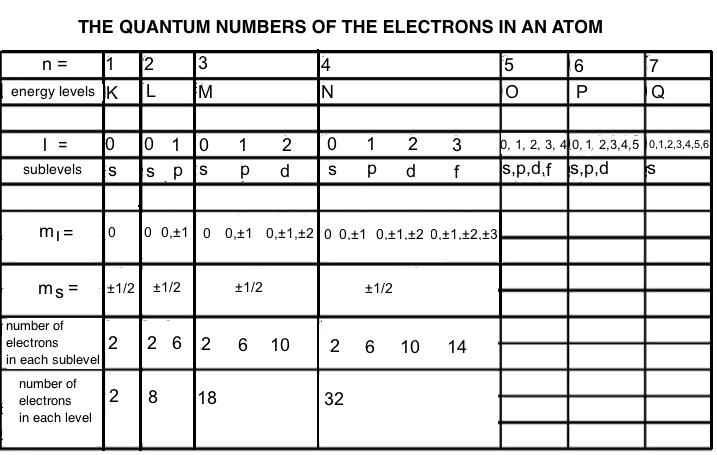 Table1
Table1
Table 2 that is an overview of the quantum numbers of the atomic electrons:
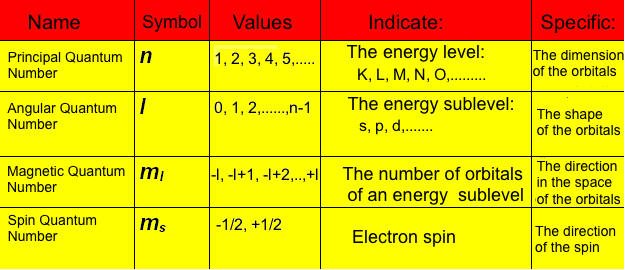 Table
2
Table
2
the
atomic
orbitals
of
kind s
and
p,
which have the following shape: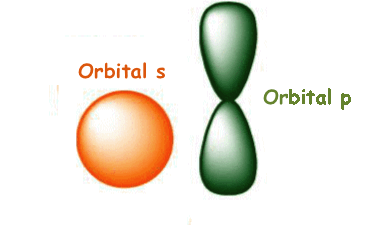
The first energy level (n = 1), coincident with the sub-level, has one atomic orbital (1s) that presents a spherical shape; it can contain a maximum of two electrons with antiparallel spin.
The second energy level (n = 2) has two sub-levels:
sub-level 2s - it has the lowest energy and it has one atomic orbital that presents a spherical shape; the radius of this atomic orbital is larger than the radius of the atomic orbital 1s. The sub-level 2s can contain a maximum of 2 electrons with antiparallel spin;
sub-level 2p - it has three atomic orbitals; each of them looks like a double opposed pear (2 lobes); the axis, along which each atomic orbital extends more, coincides with one of the cartesian axes of the space (x, y, z). The second sub-level can contain maximum 6 electrons (2 per orbital). The carbon atom contains only 2 electrons in this energy sub-level.
The second energy level, therefore, contains a maximum of 8 electrons. The carbon atom contains only 4 electrons in this energy level.
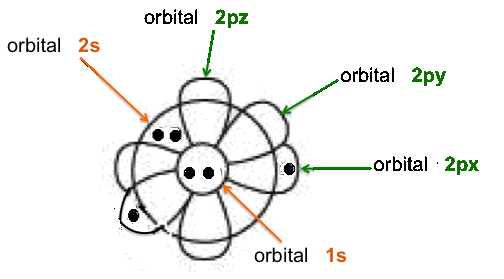 This
image shows a simplified representation of the shapes
and the filling
of all the atomic
orbitals in the carbon atom.
This
image shows a simplified representation of the shapes
and the filling
of all the atomic
orbitals in the carbon atom.
The electron distribution in an atom follows the Pauli exclusion principle that can be expressed in two different ways:
a maximum of two electrons can be simultaneously inside an atomic orbital and the electrons must have opposite spin.
inside an atom, two electrons cannot have the four quantum numbers (n, l, ml ms) equal. This means that, for example, if three quantum numbers (n, l, ml) are equal, two electrons are in the same atomic orbital, therefore the fourth quantum number (ms) must be different. Since the value of the spin quantum number is: ms = +1/2 or ms = -1/2, it is clear that the two electrons must have opposite spin.
THE CARBON IN NATURE
In nature, the carbon can be found in different crystalline forms, called allotropic forms.
The word “allotropic“ comes from the greek language: “αλλοσ” (other) and “τροποσ” (form). The allotropic forms of the carbon are:
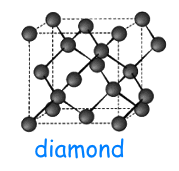
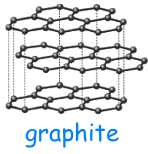
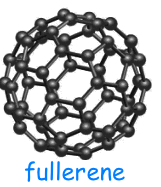
In the allotropic forms, the carbon atoms are chemically bound to each other in different ways; for this reason, these allotropic forms have different physical-chemical properties.
Graphite
is the most common structure among
the allotropic forms of carbon
 ;
it can be found in a simple pencil
;
it can be found in a simple pencil
 .
Graphite is composed of planar layers of carbon atoms that make up a
lattice with hexagonal mesh, in which each carbon atom is bound to
other three atoms.
.
Graphite is composed of planar layers of carbon atoms that make up a
lattice with hexagonal mesh, in which each carbon atom is bound to
other three atoms.
Graphite
becomes diamond
 under
high pressure and in this case the
carbon atoms are linked each other by covalent bonds;
these bonds form a tetrahedral structure and make the diamond
very resistant to the deformation.
under
high pressure and in this case the
carbon atoms are linked each other by covalent bonds;
these bonds form a tetrahedral structure and make the diamond
very resistant to the deformation.
Fullerene has a spherical structure. The most common is C60, it consists of 60 carbon atoms that form 20 hexagons and 12 pentagons, arranged in a hollow sphere.
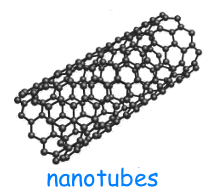
Carbon nanotubes have a cylindrical shape and can be divided in:
SWCNT (Single-Walled Carbon Nano Tube) composed of a single sheet of graphite rolled up on itself.
MWCNT (Multi-Walled Carbon Nano Tube) composed of more sheets of graphite, wrapped coaxially on each other.